Packaging of OTC Drug Tubes for Professional Samples & Kits
Flexible packaging OTC Solutions
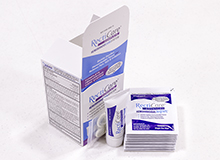
Liquipak was approached by a Pharmaceutical Industry client for packaging of OTC drug tubes. We worked with the client to co-engineer and manufacture the (over the counter) OTC drug tubes highlighted here. After a comprehensive development process, tube counting and packaging was performed in our ISO 9001:2015 certified, FDA registered facility. A vibratory bowl system with an electronic eye was utilized for counting and accumulating the tubes. Accumulated tubes are then deposited into the dispensing cartons along with a printed insert and a plastic applicator. Each finished tube measured 3″ in length, 3/4″ in width, and 0.50″ thick
To ensure the highest product quality, we performed digital test stand compression testing, digital gram-scale weigh testing, component and batch traceability, labeling and product accountability, and AQL final inspection. This project was completed in 2 weeks and consisted of more than 100,000 units.
Additional details of this counting and packaging of OTC drug tubes project can be found in the table below or you can contact us to learn more.
Highlights of this Counting and Packaging of OTC Drug Tubes for Professional Samples and Kits Project
| Counting & Packaging Capabilities Applied/Processes | Primary: Co-Engineering
Production – Tube Counting |
Secondary: Custom Packaging – Printed inserts and plastic applicators are added to each dispenser. |
| Equipment Used | Vibratory bowl system with electronic eye for counting and accumulating tubes | |
| Overall Part Dimensions | Length: 3″ Width: 3/4″ |
Thickness: 0.50″ |
| Material Used | Customer Supplied Plastic single use tubes of OTC drug | |
| Packaging | Vibratory bowl counting, accumulating and delivery of tubes into dispenser cartons. | |
| Additional Facts | A lot-code and an expiration date are applied to each dispenser. | |
| In process testing/inspection performed | Digital test stand compression testing Digital gram-scale weigh testing Component and batch traceability |
Labeling and product accountability AQL final inspection |
| Industry for Use | Pharmaceutical Industry | |
| Volume | 100,000 + | |
| Delivery/Turnaround Time | 2 weeks | |
| Delivery Location | Alma, Michigan | |
| Standards Met | FDA registered Drug Repackager cGMPs Customer’s packaging specifications |
ISO 9001:2015 Certified QMS Internal quality system requirements |
| Product Name | Tubes of OTC drug product for professional samples and kits |